CLINICAL DATA
A promise backed with evidence. Proven results including a large RCT published in The Lancet Diabetes & Endocrinology1 and supported with additional solid scientific and clinical data.1-29
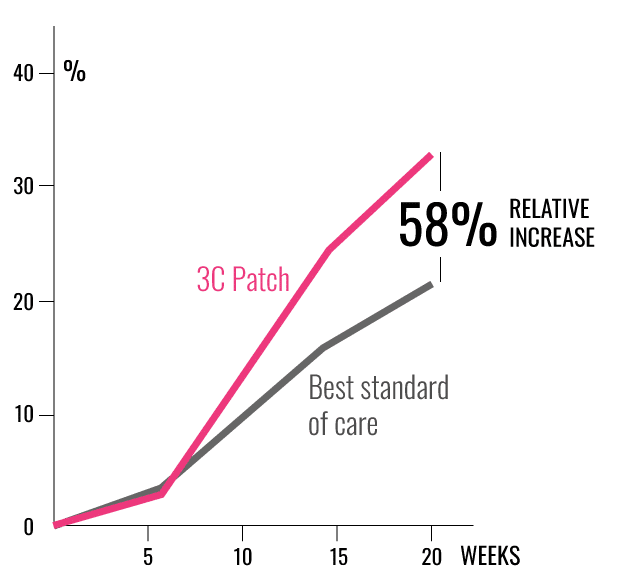
IMPROVED CHANCES OF HEALING
%
COMPLETE WOUND CLOSURE
RESULTS FROM THE INTERNATIONAL MULTICENTER RCT ON 269 HARD-TO-HEAL DIABETIC FOOT ULCER PATIENTS
STUDY DESIGN1
An independent multicenter, multinational, observer blinded, investigator driven Randomized Controlled Trial.1
The RCT study was sponsored by Nottingham University Hospitals NHS Trust, Nottingham UK. The funder, Reapplix ApS, had no role in study design, study performance, data collection, data analysis or data interpretation.
INTERVENTION1
3C Patch® vs. “Best standard of care.” Intervention period of 20 weeks followed with a 6-week observation period.
PATIENTS1
- 595 were recruited from 32 specialist foot care centers in UK, Sweden and Denmark.
269 were randomized between August 2013 and May 2017. - The groups were well matched at baseline and consisted of those in most need of new treatment, those with “hard-to-heal” Diabetic Foot Ulcers (DFU´s).
- “Hard-to-heal” defined rigorously as having DFU´s that did not reduce in area more than 50% over a 4 week run-in period despite best standard of care including offloading where appropriate.
KEY EFFICACY DATA1
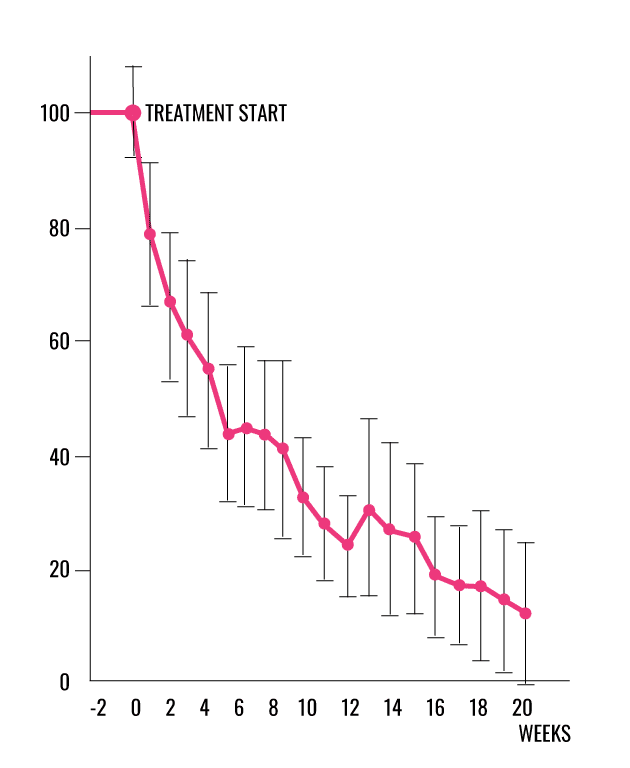
- 89% more likely to heal using a linear regression model, defined in the protocol, to account for the wound size distribution between the control and treatment arms. (CI 1.09 – 3.31, p =0,0237) giving odds-ratio 1.89.1
- 58% more patients healed from the intervention compared with best standard of care using ITT giving odds ratio 1.58.1
- 34,1% (45/132) healed by 20 weeks in the intervention group compared with 21,6% (29/134) in the controls (p=0.0235).1
KEY SAFETY DATA1
- No differences in adverse events or serious adverse events between groups.
- No device related serious adverse events.
- No differences in new anaemia despite weekly blood sampling.
RESULTS FROM THE MULTICENTER COHORT STUDY
STUDY DESIGN2
Prospective multicenter cohort study of patients with hard-to-heal Wagner grade 1-2 diabetic foot ulcers.
INTERVENTION2
“Patch” vs. “No Patch” but with same treatment strategies including off-loading, antibiotics and debridement.
PATIENTS1
- 60 patients recruited.
- Adult patients, with at least one full-thickness diabetic ulcer, classified by the investigator as Wagner grade 1 or 2.
- Duration of ulcer more than 6 weeks and a maximal area of 10 cm2.
- Only included ulcers that did not reduce in area more than 40% over a 2 week run-in period, to ensure “hard-to-heal” ulcers were studied.
- 16 patients were not included after the run-in period, mainly due to healing. Five patients did not complete the study.
- The study did not exclude based or wound depth of wound grade; down to tendon and down to bone was also allowed.
KEY EFFICACY DATA2
- 39 patients included in per protocol analysis.
- 36% achieved complete epithelisation at 12 weeks.
- 59% achieved complete epithelisation at 20 weeks.
- 73% of ulcers healed within 20 weeks for patients with ulcer duration less than 6 months.
- Larger ulcer area reduction during the first two treatment weeks for healers as compared to non-healers.
- Healing rates at 20 weeks were significantly higher in the third of the patients with the shortest ulcer duration as compared to the third with the longest duration (73.3% vs. 26.7% p=0.026).
KEY SAFETY DATA2
None of the adverse events were judged to be related to the patch treatment.
CONCLUSIONS2
- More than 70% of ulcers with duration between 8-25 weeks healed.
- 59% of the ulcers healed at 20 weeks and the mean time to complete healing was 11 weeks despite the average wound duration of 56.5 weeks at inclusion.
- More than one third of non-healing ulcers with a duration of more than one year healed during the 20 week period.
- The patch, characterized by its 3-layer composition is a well-tolerated treatment feasible for clinical practice and has a potential in the armamentarium of the DFU.
WOUND AREA CHANGE